Streamlined FDA Registration
for Hand Sanitizer Manufacturing
Manufacturing Approval During the Public Health Emergency
The current public health emergency has created a widespread shortage of alcohol-based hand sanitizers. Hand sanitizers are regulated as an over the counter (OTC) medication and are overseen by the FDA. Firms currently involved with packaging, production of ethyl alcohol and isopropyl alcohol, raw materials, packaging, and Current Good Manufacturing Practices (cGMP) requirements have an immediate opportunity to enter this market. Leveraging our extensive expertise in FDA cGMP regulations, WorkingBuildings Clinical (WBC) can help guide your firm through the FDA establishment and drug registration process.
We conduct a thorough review of the rapidly changing FDA regulations and support your firm through the FDA registration framework. This allows you to quickly market alcohol-based hand sanitizer you manufacture or purchase from outsourced manufacturers that meet the requirements of the emergency regulations.
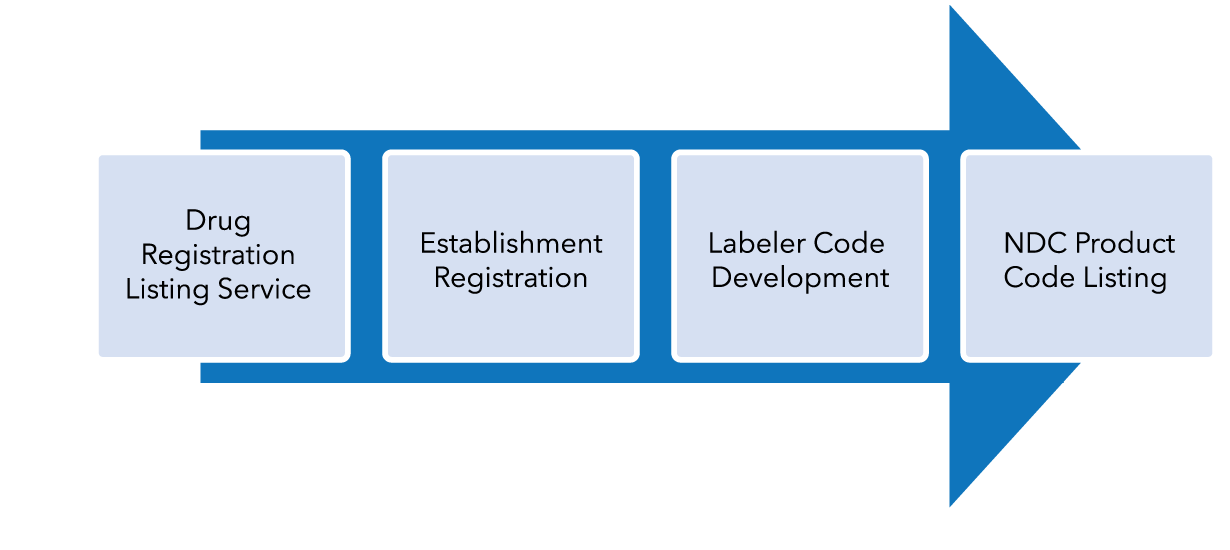
Firms interested in manufacturing hand sanitizer during the public health emergency must follow specific requirements from the World Health Organization which specify either ethyl alcohol or isopropyl alcohol as the active ingredient. The steps required for a manufacturer to enter commercial distribution during the current public health emergency include the following steps:
FDA Emergency Registration Process
FDA Product Listing After the Public Health Emergency
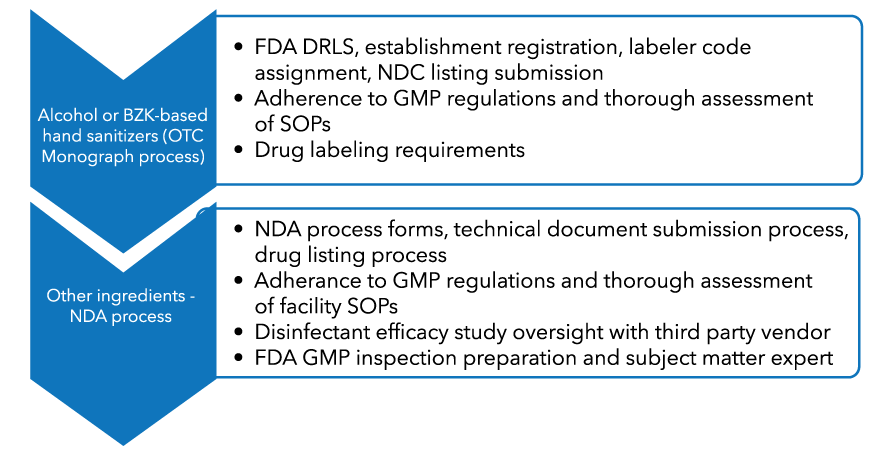
Once FDA provides notification that the public health emergency is over, firms interested in continuing to manufacture, package, or label hand sanitizer product will fall under the oversight of FDA as a drug manufacturing facility and will be subject to Current Good Manufacturing Practices (cGMP) requirements. Hand sanitizer products listed in the FDA OTC Monograph (Benzalkonium chloride, Ethyl alcohol or Ethanol (60 to 95%) and Isopropyl alcohol (70 to 91.3%) do not require a lengthy FDA pre-approval process. Hand sanitizer products containing active ingredients other than these three will require a New Drug Application (NDA) process to be submitted with the FDA.
WBC can assist your facility with the following steps to ensure FDA compliance for your hand sanitizer product:
FDA cGMP Registration Requirements